Self-test for Self-application
RAPID TESTS FOR EVERYONE
Finally, you can test yourself where and when it´s most convenient for you: with our test kit, you can make a qualitative in vitro diagnostic of the SARS-CoV-2 antigen by means of a simple smear in the anterior nose area – even if you have no medical/clinical experience. Thanks to precise step-by-step instructions, carrying out the test is dead easy.

Checkliste
What’s special about our Coretests® for Self-user?
- CE marking by notified body (CE 1434)
- BfArM listed under AT1388/21 (Link)
- Smear in the anterior nose area
- No environmentally harmful substances
- Clinical sensitivity: 95.11 %
- Clinical specificity: 100 %
- Easy handling proven by a German usability study
- Prefilled buffer solution
- Evaluated according to the criteria of the RKI and PEI
- Available in 1-softpacks
- Detection of viral mutations including omicron
- Multi-language instruction for use: DE, EN, FR, ES, IT, NL
AT A GLANCE
COVID-19 Ag TEST for self-application

15 Minuten
test time
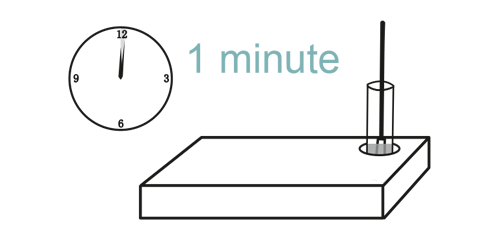
The test result is available within 15 minutes.

2ºC–30ºC
storage
Easy handling and long? shelf life due to extended temperature range.

NASAL SWAB
Sample type
Smear in the anterior nasal cavity, nasopharyngeal and pharyngeal.
TEST PROCEDURE
CORETESTS®-COVID-Ag TEST for self-use
APPLICATION/TRAINING VIDEO
APPLICATION STEP BY STEP
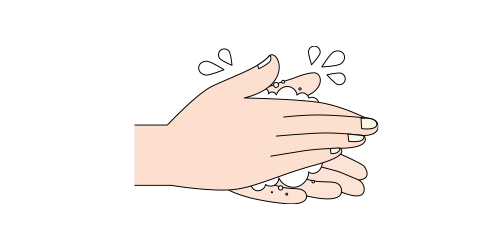
Step 1
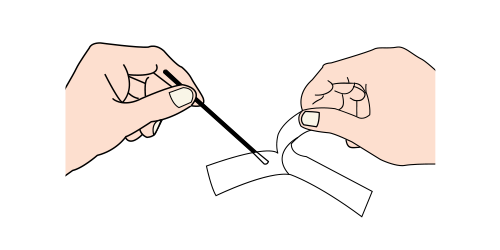
Step 2
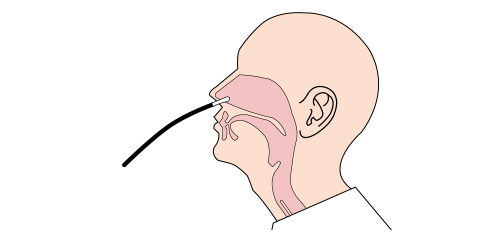
Step 3
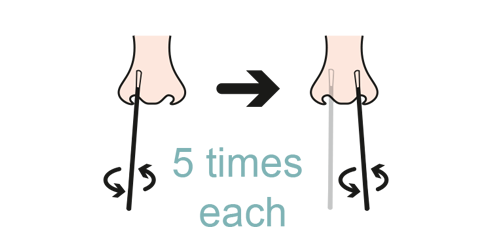
Step 4
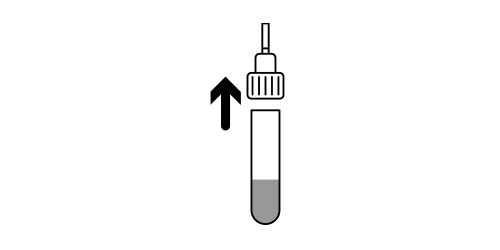
Step 5

Step 6
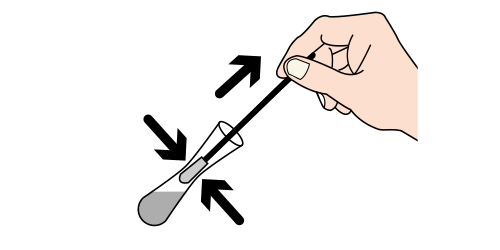
Step 7
Step 8
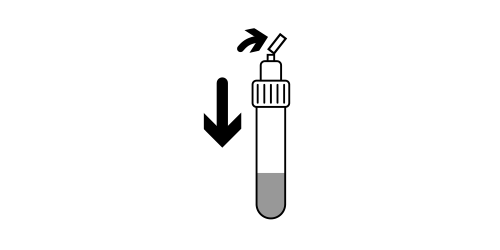
Step 9
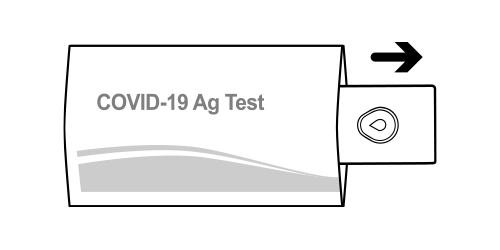
Step 10
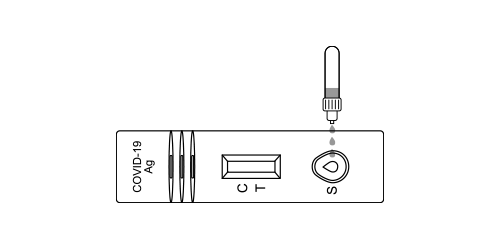
Step 11

Step 12
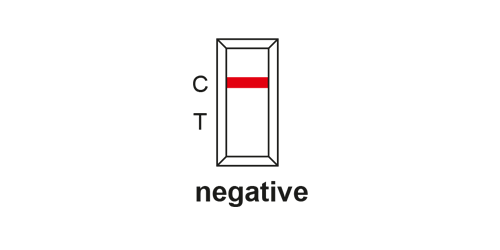
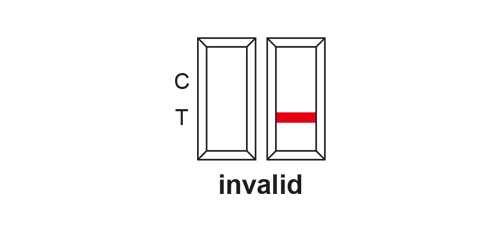
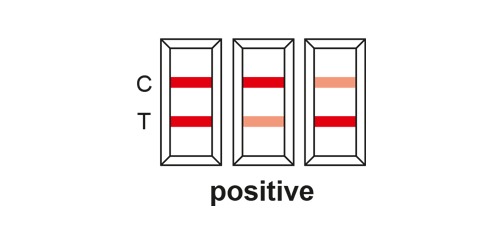
EVALUATION
FREQUENTLY ASKED QUESTIONS
Regarding the Coretests®-COVID-19 AG rapid tests for self-application
100% reliability cannot be guaranteed by a rapid antigen test; however, this can also occur with other test methods, such as PCR tests. Using the PCR test as the sole diagnostic method quickly reaches economic proportions (laboratory equipment, costs etc.) that the global health system cannot afford due to the number of infections. COVID-19 rapid tests provide a useful supportive alternative.
COVID-19 rapid tests can be carried out cost-effectively, decentrally and by any healthcare professional – or even as self-tests by the general public. No additional equipment is necessary, as is the case with a complex PCR smear test.
Currently, COVID-19 rapid tests are used in nursing homes, hospitals or schools for regular testing of staff and residents. In test centers, all citizens can be tested by medical professionals. Self-tests can provide additional security in specific, every-day situations – for example, before visiting friends and family, when eating out, or before visiting the theatre or cinema. If a COVID-19 rapid test is positive, the result must be confirmed by a PCR test.
The sensitivity of a diagnostic test procedure indicates the percentage of sick patients who actually detect the corresponding disease by test application. This is calculated as a quotient of right-positive test results and the sum of right-positive and false-negative test results. The higher the sensitivity, the more reliably the disease is diagnosed.
The specificity of a diagnostic test procedure indicates the probability with which healthy test persons are also recognized as healthy in the test. This is calculated as a quotient of indeed-negative test results and the sum of false-positive and right-negative test results.
In order to ensure the reliable detection of a transmission-relevant infection, the detection limit of the antigen tests should be based on the data collected so far by the RKI. The detection limit describes the extreme value of a measurement method up to which the corresponding measured quantity can barely be detected.